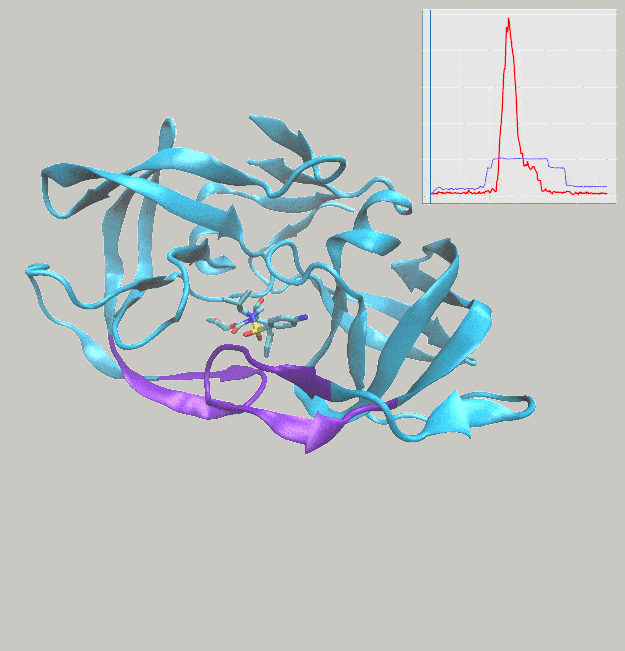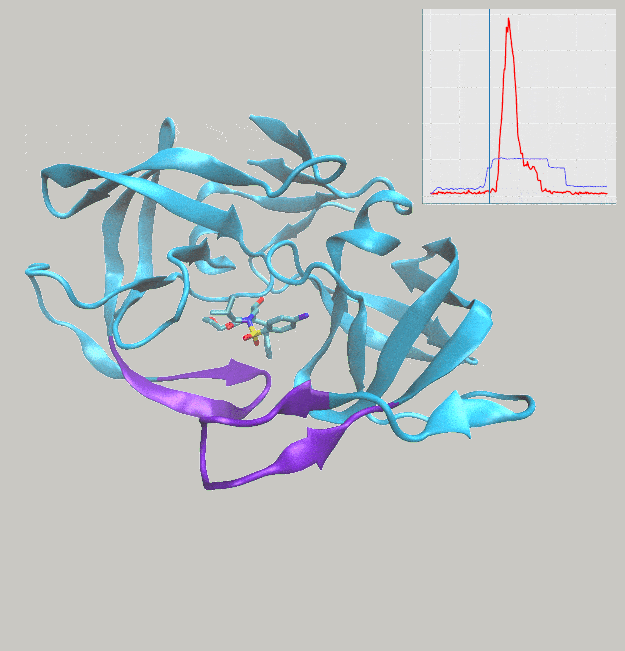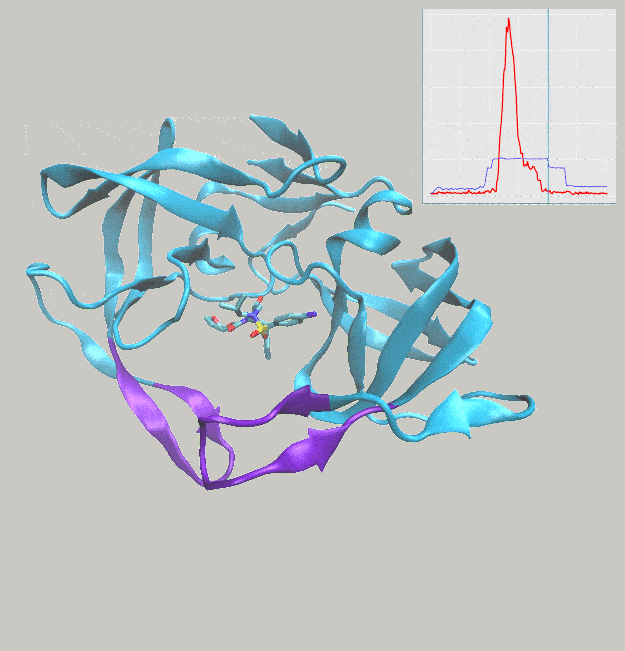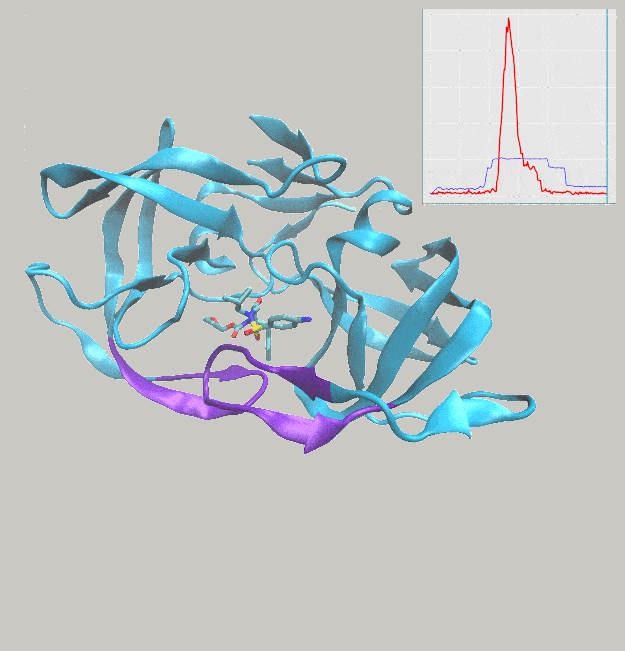iMD-VR for flexible protein-ligand docking
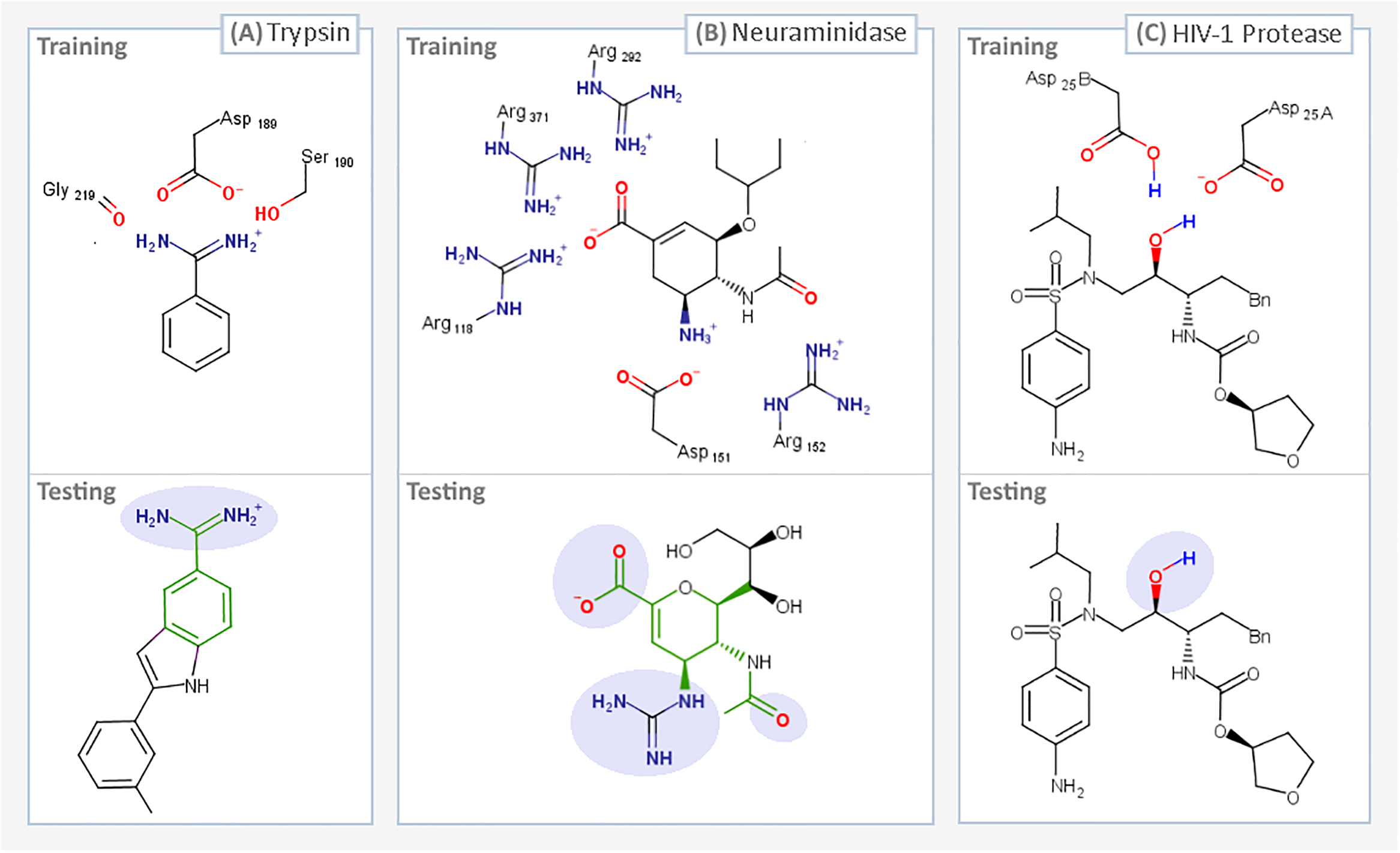
We’ve just published a paper describing the use of interactive molecular dynamics in virtual reality (iMD-VR) for carrying out flexible protein-ligand docking, demonstrated through experiments carried out docking drug molecules into the binding pockets of trypsin, neuraminidase, and HIV-1 protease.
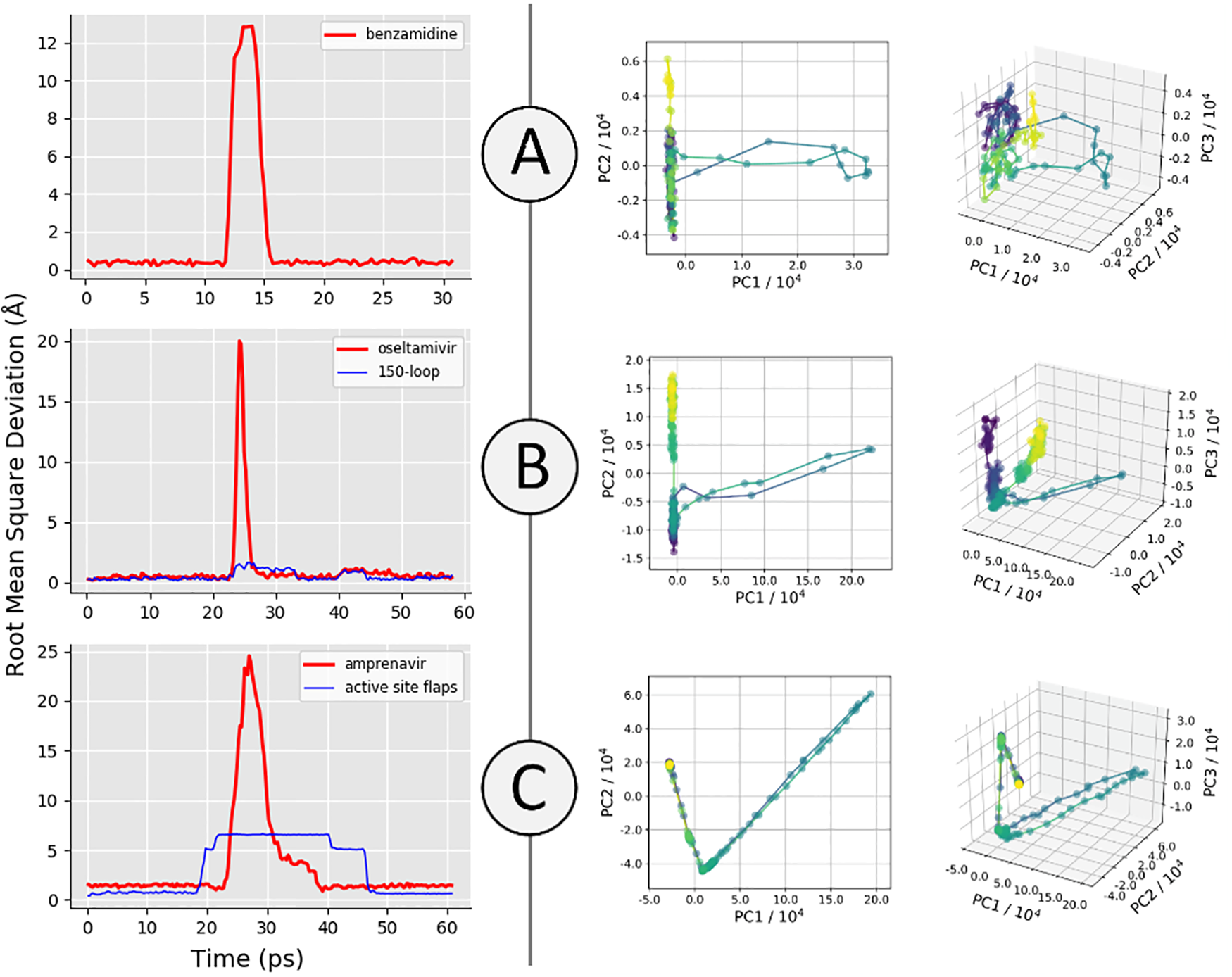
A major aim of the work was to investigate whether iMD-VR tools afforded sufficient control for users to accurately carry out the detailed atomic manipulations required to dock flexible ligands into dynamic enzyme active sites and recover crystallographic poses. The results show that both iMD-VR experts and novices alike were able to recreate respective crystallographic protein-ligand binding poses within 5–10 minutes – and the majority were able to recover binding poses within 2.15 Å RMSD of the crystallographic binding pose!
These results suggests that iMD-VR offers an interesting new approach for simulating drug docking and generating binding hypotheses.
Abstract
Simulating drug binding and unbinding is a challenge, as the rugged energy landscapes that separate bound and unbound states require extensive sampling that consumes significant computational resources. Here, we describe the use of interactive molecular dynamics in virtual reality (iMD-VR) as an accurate low-cost strategy for flexible protein-ligand docking. We outline an experimental protocol which enables expert iMD-VR users to guide ligands into and out of the binding pockets of trypsin, neuraminidase, and HIV-1 protease, and recreate their respective crystallographic protein-ligand binding poses within 5–10 minutes. Following a brief training phase, our studies shown that iMD-VR novices were able to generate unbinding and rebinding pathways on similar timescales as iMD-VR experts, with the majority able to recover binding poses within 2.15 Å RMSD of the crystallographic binding pose. These results indicate that iMD-VR affords sufficient control for users to carry out the detailed atomic manipulations required to dock flexible ligands into dynamic enzyme active sites and recover crystallographic poses, offering an interesting new approach for simulating drug docking and generating binding hypotheses.